Bioanalytical Services for Every Phase of Drug Development
Our bioanalytical team excels in comprehensive bioanalytical services, including assay and method development, molecular biology, LC-MS, ligand binding assays (LBA), immunogenicity testing, biomarker analysis, microsampling, flow cytometry services, and more, supporting your journey from discovery through Phase IV. Strategically located for integrated service delivery, we focus on delivering quality data for TK, PK, and PD analyses, critical for preclinical and clinical study success.
Precision Bioanalytical Services for Drug Development
We offer a full range of bioanalytical services conducted in state-of-the-art, purpose-built laboratories at our locations in the U.S. and Canada. Our precision bioanalytical services as standalone work or as part of a service package.

HIGHLY TRAINED
SPECIALISTS
SAMPLES CAPACITY
PER YEAR
ASSAYS COVERING
650+ MOLECULES
Comprehensive Bioanalytical Expertise for Precise Results
Altasciences' bioanalytical labs excel in assay development, backed by a team of over 260 scientists. Our capabilities span mass spectrometry (LC-MS/MS), ligand binding assays (LBA), immunogenicity testing, flow cytometry, microsampling, ELISpot, biomarker analysis, and more, with a robust database. Specializing in diverse biological matrices and state-of-the-art laboratories, we are fully equipped to support internal and external studies.
-
 Ligand Binding Assays
Ligand Binding Assays -
 Translational Biomarkers
Translational Biomarkers -
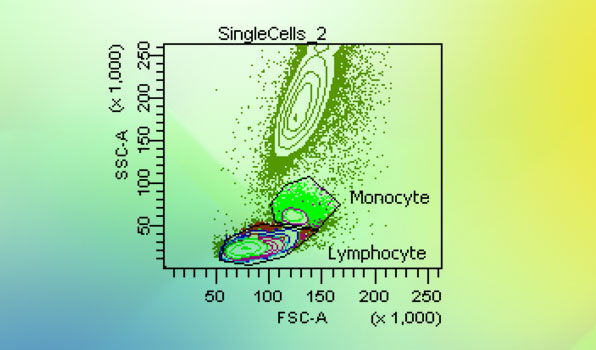 Flow Cytometry
Flow Cytometry -
 Oligonucleotides
Oligonucleotides -
 Platforms
Platforms -
 Microsampling
Microsampling -
 Immunogenicity Testing
Immunogenicity Testing -
 Cell and Gene Therapy Development
Cell and Gene Therapy Development -
 LC-MS and Hybrid LC-MS Bioanalysis
LC-MS and Hybrid LC-MS Bioanalysis -
 Sample Management Solutions
Sample Management Solutions
Bioanalytical Laboratories—Expertise Across Modalities
-
Our bioanalytical laboratories support LC MS/MS and ligand binding assays for small and large molecule programs. From discovery through Phase IV, we can support a full range of bioanalytical services with our extensive assay database, expertise in PCR, flow cytometry, oligonucleotides, microsampling, and more to support your bioanalytical needs.

-
 Flow Cytometry Services
Flow Cytometry Services -
 Bioanalytical Solutions for Immunogenicity Testing
Bioanalytical Solutions for Immunogenicity Testing -
 Translational Biomarker Assays
Translational Biomarker Assays -
 Bioanalytical Expertise for Immunomodulatory Drugs
Bioanalytical Expertise for Immunomodulatory Drugs -
 Bioanalytical Expertise for ADCs
Bioanalytical Expertise for ADCs
Bioanalysis - FAQs
Are your bioanalytical services adaptable to different drug development program requirements?
Our bioanalytical services are entirely flexible. You can partner with us for standalone analysis of your samples, or we can conduct our precision bioanalytical services as part of a full drug development package. We offer LC-MS/MS, ligand binding, or hybrid processes for your large or small molecules, including specialized large molecule LC-MS and a dedicated ligand binding lab space, for preclinical and clinical samples. We also offer nonclinical laboratory services, such as anatomic and clinical pathology, and toxicokinetics.
How long has Altasciences been providing bioanalytical services?
We have been delivering excellence in bioanalytical services for more than 30 years, and we have more than 200 experts working at our labs. With shifts running 24/7 if needed, our laboratory teams are able to process as many as 60,000 samples per month.
How does Altasciences ensure optimal communication between their clients, and their bioanalytical services teams?
A Bioanalytical Principal Investigator manages every project, and works hand in hand with you through every step of the project. We ensure accurate and complete capture of your requirements, so that you only must Tell Us Once™.
Do your laboratories have the capability to handle specialized projects such as microsampling, gene therapy, oligonucleotides, etc?
Yes, we can accommodate your bioanalytical or preclinical needs for biologics, gene therapies, oligonucleotides and other precision requirements. We have state-of-the-art equipment, and highly skilled experts for your specialized needs such as large molecule LC-MS/MS, ligand binding, gene therapy analysis, and microsampling. We also have designated containment Level 2 areas for work with risk group 2 pathogens.




