Comprehensive Immunogenicity Assays and Testing Solutions
Immunomodulators are transforming medical treatment with their profound impact on cancer, autoimmune, infectious diseases, and vaccine development. Each class of immunomodulator has a defined complexity and mechanism of action; thus, the appropriate bioanalytical program will need to be carefully designed for the drug type as well as the intended purpose of the clinical study. Considerations include the appropriate PK and PD endpoints required for the study, and their regulatory and bioanalytical specifications.
We offer comprehensive bioanalytical services for the therapeutic development of immunomodulatory drugs, from immunomodulator analysis to immunogenicity testing; our teams have experience handling pathogenic/infectious samples, supporting studies in chronic diseases.
We tailor and validate fit for purpose assays to your program’s requirements.
We have state-of-the-art bioanalytical platforms for multiple forms of immunomodulatory drug analysis, all with high sensitivity and specificity of detection. Discover some additional key features of each platform.
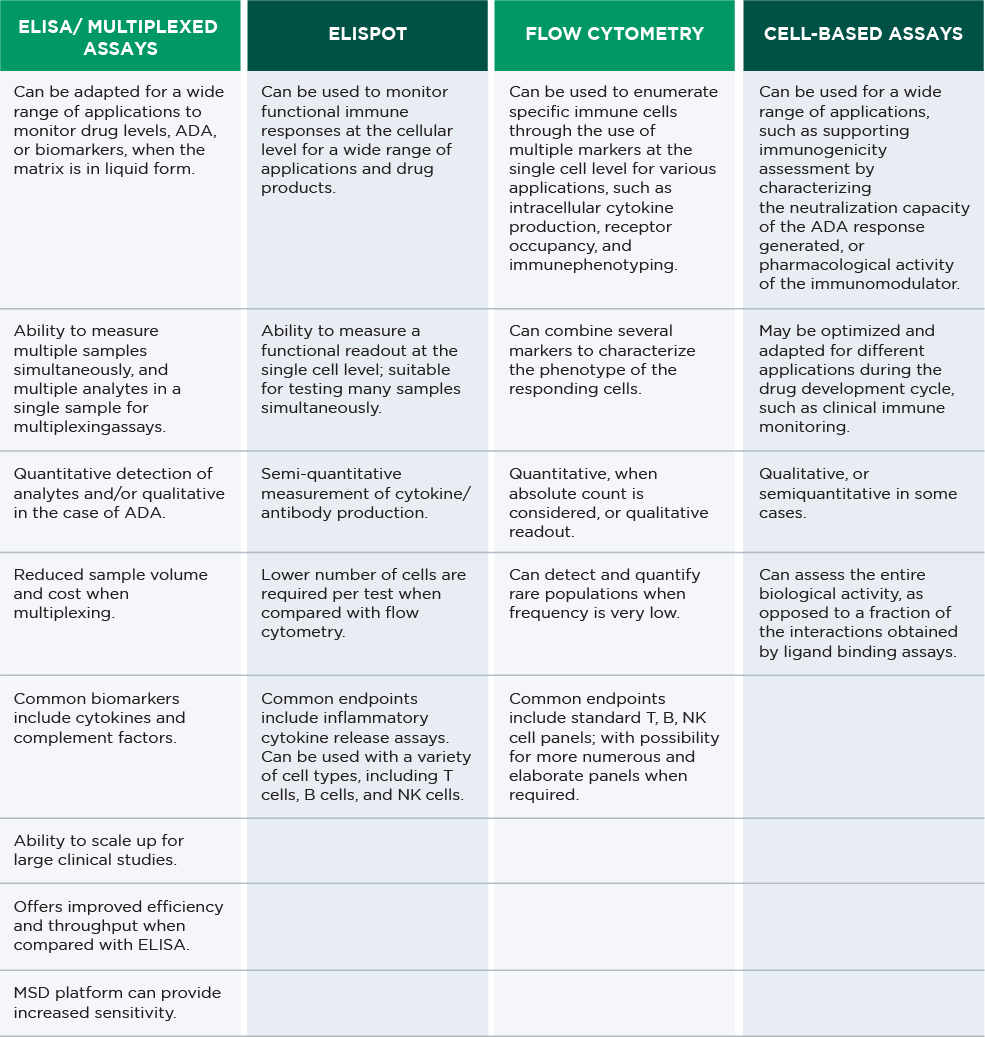
IMMUNOGENICITY TESTING CAPABILITIES
Whether you're developing a vaccine, biologic or gene therapy, immunogenicity serves as an important assessment. It gauges the antibodies produced in response to your drug, helping you confidently determine efficacy and safety
We provide the following:
- Bioanalysis to support preclinical and clinical immunogenicity studies: qualified versus fully validated methods
- Bridging ECL assay and direct ELISA assay
- Experience with interference issues, mitigation strategies, and/or binding components
- Acid dissociation, SPEAD, ACE, PandA
- Physical separation by size exclusion or magnetic protein A/G + L
- In-house labeling of antigen with biotin and sulfo-TAG
- Positive control generation service
- Statistical analysis supported by biostatisticians
- Validations per the 2019 FDA guidance
We have experience with:
- drug-mAb, PEGylated proteins, endogenous analogs, peptides, fusion proteins, oligonucleotides, gene therapy, ADCs, PDC, bispecifics, and more;
- interference issues from the drug, drug target and/or binding components, and various mitigation strategies;
- pre-existing antibodies—the appropriate cut point approach will be determined based on the prevalence and magnitude of responses;
- positive control generation and characterization, mAb, and polyclonal sera;
- several study populations: healthy and patient (immuno-oncology, oncology, malabsorption disorder);
Assay Types
Our team has extensive experience developing and validating a wide range of characterization assays, including assessment of cross-reactivity to endogenous proteins. In addition to being able to titrate your samples, we can also support binding specificity assessment, isotype determination and perform NAb assays, like competitive ligand binding or NAb cell-based assays, depending on the mechanism of action of your drug.
VALIDATION OF IMMUNOGENICITY ASSAYS TO SUPPORT NONCLINICAL AND CLINICAL STUDIES
Nonclinical Studies
Altasciences provides nonclinical immunogenicity method qualification or validation based on industry recommendations and study-specific needs. The choice between qualification and validation depends on the drug development stage and GLP status. ADA method qualification includes basic parameters in fewer replicates, and validation comprises the screening assay with optional titration or confirmation assays. We customize the process to support nonclinical study requirements.
Clinical Studies
Immunogenicity assays in drug development evolve with program progression. Nonclinical assays can be qualified with minimal requirements, while clinical support assays must meet well-defined, regulation-compliant standards. Achieving FDA-recommended sensitivity levels, such as 100 ng/mL for anti-drug antibody assays, requires scientific expertise to investigate sensitive and specific measurement of analytes in complex biologic matrices. Interference challenges in immunogenicity assays, especially in disease-type populations, involve factors like the drug, rheumatoid factor, and co-administered drugs. Successful strategies have addressed interference over the years, but access to disease-type matrices for method validation can be limited, raising questions about their representativeness due to study protocol criteria. Altasciences has experience with study populations including healthy normal volunteers, Asian populations for ethnobridging, and patients with various conditions.
Related Resources
-
 Bioanalytical Expertise for ADCs
Bioanalytical Expertise for ADCs -
 Translational Biomarker Assays
Translational Biomarker Assays -
 Bioanalytical Solutions for Immunogenicity Testing
Bioanalytical Solutions for Immunogenicity Testing -
 Bioanalytical Expertise for Immunomodulatory Drugs
Bioanalytical Expertise for Immunomodulatory Drugs -
 The Altascientist—Quantification of ASOs
The Altascientist—Quantification of ASOs -
 eBook: ASOs—Expert Interviews, Case Studies, and More!
eBook: ASOs—Expert Interviews, Case Studies, and More! -
 Overview of LBA and LC–MS/MS analysis of ASOs
Overview of LBA and LC–MS/MS analysis of ASOs -
 Poster: Dev. of a Surrogate CSF Matrix for Quantitative Analysis of ASOs
Poster: Dev. of a Surrogate CSF Matrix for Quantitative Analysis of ASOs
LEARN MORE ABOUT OUR Laboratory SERVICES
Click below to explore more of Altasciences’ bioanalytical solutions.