ONE PARTNER FOR ALL EARLY-PHASE DRUG DEVELOPMENT
Drug development is complex: every step demands precision, speed, and reliability. And managing multiple vendors for the different R&D stages slows you down.
Altasciences simplifies the process. As the only CRO/CDMO offering end-to-end early-phase development, we take your molecule from lead candidate selection to clinical proof of concept—and beyond. No duplicate admin work. No communication gaps. Just a seamless path forward with a single partner.
In a world where time and resources are precious, Altasciences is the partner you can trust to deliver results—quickly, seamlessly, and with the highest standards of safety and excellence. Let us take care of your R&D, so you can focus on what matters most: bringing your innovative therapies to the world.
THE CLEAR CHOICE
You are on the brink of a breakthrough—an innovative therapy with the potential to change lives. Your science is strong, and your vision is clear, but the path from discovery to early-phase development is complicated… Until now.
At Altasciences, we simplify your journey. Unlike traditional CROs that handle only certain aspects of early-phase drug development, we can support you through nonclinical safety testing, clinical pharmacology, and bioanalysis, as well as drug formulation and manufacturing—all under one contract. With streamlined workflows, coordinated handoffs, and seamless data sharing, our clients experience faster timelines, reduced administrative burden, and greater confidence in every phase of development.
See how we compare with other CROs and CDMOs in the industry:
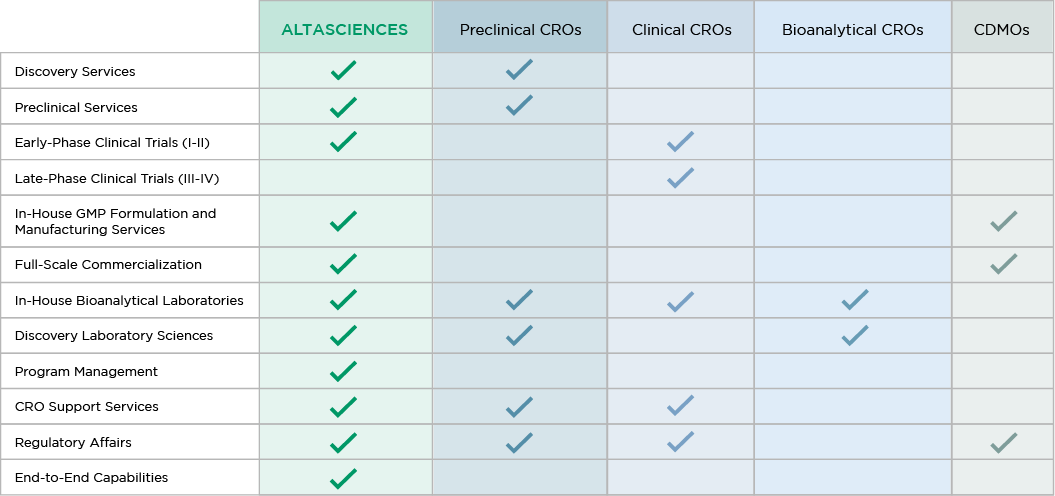
Planning a Drug Development Program? Altasciences Can Help.
With Altasciences’ approach, your molecule will advance quickly through major milestones to deliver valuable data, empowering you with robust insight to make informed, and more complete, decisions about your drug candidates, sooner. We do this through transparent collaboration across all stages of early-phase development, with comprehensive research services and integrated scientific teams—eliminating the barriers that typically slow down drug development.
We’re far from the standard—watch the series:
YOUR DRUG DEVELOPMENT JOURNEY STARTS HERE
Questions? Fill out this form and one of our experts will reach out to discuss your study and/or program needs.
HOW CAN ALTASCIENCES ACCELERATE YOUR DRUG DEVELOPMENT?
We treat drug development as a continuum—not a series of isolated steps.
Our synchronized early-phase pathway, powered by proprietary communication and scheduling platforms, streamlines outsourcing and optimizes timelines. By running R&D activities in parallel where possible, we eliminate delays and reduce costs. This proactive approach helps us anticipate challenges and safely accelerate your timelines by up to 40%.
A SMARTER APPROACH TO EARLY-PHASE DRUG DEVELOPMENT
The standard way of outsourcing drug development involves working with multiple service providers, leading to bottlenecks, miscommunication, and delays—not to mention the added administrative burden on you.
Altasciences offers a smarter solution. By centralizing early-phase drug development, we streamline processes, improve efficiency, and reduce risks. With one partner managing your program from start to finish, you gain speed, clarity, and a seamless experience.
NEVER REPEAT YOURSELF AGAIN
Have you ever found yourself repeating your study history, details, goals, and preferences at every new study phase or when you meet a new member of your study team? With Altasciences, those days are over!
Tell Us Once™ is our commitment to proactively sharing your preferences, requirements, drug information, and study results across all our internal teams. We have the people, the processes, and the integrated systems in place to provide you with a seamless customer experience. Like we always say, you only have to Tell Us Once™; we’ll take care of the rest.
WHY CHOOSE US FOR YOUR PROGRAMS
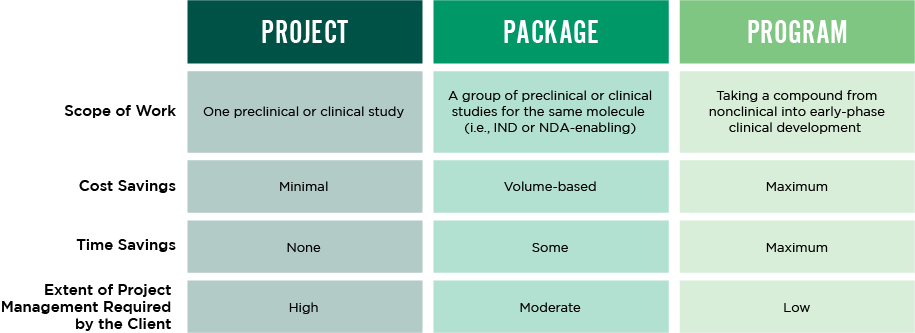
You can partner with Altasciences for a single study, multiple studies, or a full end-to-end program—we’re flexible like that. Partner with us for multiple studies, and you will experience all the benefits Proactive Drug Development has to offer. As your molecule progresses, we build on existing knowledge to streamline your roadmap and save time and costs by running key activities in parallel. Our integrated approach also eliminates the hassle of managing multiple CROs and CDMOs, so you can focus on advancing your drug candidate.

Dear Standard CRO,
This video campaign was designed with a touch of humor and is not intended to accurately portray our fellow CROs and CDMOs. We have great respect for their contributions to scientific research and recognize that, like us, they are committed to supporting the development of life-saving therapeutics for the benefit of patients.