Preclinical pharmacology studies
Our preclinical pharmacology services are specifically designed to optimize drug efficacy, safety, and dosing, ensuring that your compound has the best chance of advancing to clinical trials. We accelerate the development of your safe and effective therapies by offering customized solutions, advanced technologies, and expert guidance that align with your specific goals, timelines, and budgets―ensuring you receive the most relevant data for your research to propel your project forward.
Contact us to learn how our preclinical pharmacology services can support your drug development program.
Preclinical Pharmacology Expertise for Early-Phase Drug Development
Altasciences brings a wealth of expertise and experience in preclinical pharmacology services that truly sets us apart from other CROs. We collaborate closely with you on study design, incorporating meaningful endpoints and a clear path forward. Whether you're a biotech startup, mid-sized company, or a large pharmaceutical firm, our proven expertise and commitment to delivering high-quality results make us the right partner. We also provide expert guidance throughout the preclinical phase to help refine study design and improve overall outcomes.
Our team of seasoned pharmacologists understand the complexities of drug behavior in biological systems, allowing us to optimize your research program. We have successfully conducted hundreds of studies, including toxicology and pharmacokinetics/pharmacodynamics, across various therapeutic areas, such as:
- cardiovascular diseases
- metabolic diseases
- infectious diseases
- gene therapy for multiple indications
- inflammation and autoimmune diseases
- musculoskeletal diseases
- neurology
- ocular diseases
- oncology and immuno-oncology
- rare diseases
- respiratory diseases
Our pharmacological testing services, full bioanalytical support, and preclinical safety testing services can predict the biological effects of your new therapeutic entities and provide you with the data you need for your regulatory submissions.
Our team has developed a diverse range of in vivo study models spanning numerous disease indications. These resources can help you plan your preclinical pharmacology studies and enhance comprehension of your innovative therapies from both efficacy and safety perspectives.
Our preclinical pharmacology services include:
- in vivo drug efficacy, safety, and combined efficacy/safety studies to assess the therapeutic potential of your compounds in animal models
- pharmacokinetic studies to investigate the absorption, distribution, metabolism, and excretion (ADME) of your drugs
- safety pharmacology evaluations to assess the potential cardiovascular, respiratory, and central nervous system risks and safety profiles of your compounds
In addition, we have various preclinical pharmacology models established in multiple species to help with evaluating your test article for specific indications. Many test articles have potential or recognized targets that require expert evaluation to fully realize the scope of their on-target and off-target effects.
A Comprehensive Approach: Efficacy and Safety Testing for Drug Development
Our highly qualified teams of surgeons, veterinarians, and quality assurance professionals offer solid technical, scientific, and regulatory support for various procedures designed to characterize your test article’s pharmacological effects.
A typical preclinical pharmacology study involves the following stages, though each study may vary depending on the specific needs of your drug and the disease being targeted.
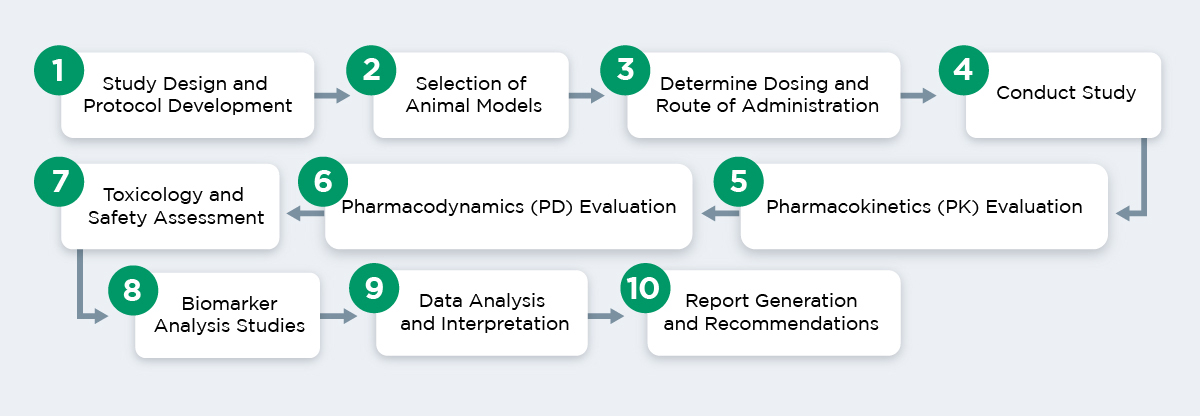
Our capabilities also include specialized assays such as liver biopsy, bone marrow aspirates, serial CSF samples, full cardio-respiratory capabilities, and central nervous system evaluations. Appropriate pharmacodynamic biomarkers and histopathology analysis also support our efficacy models.
In addition, collaboration with our integrated bioanalytical, clinical, and manufacturing teams fosters open communication, allowing us to adapt to any changes in your study requirements and deliver timely updates.
Advanced Methodologies, Technologies, and Regulatory Guidance
We recognize the importance of generating valuable preclinical data to help predict clinical outcomes. By utilizing advanced instrumentation and innovative methods, we provide accurate, reliable, and translatable results. From high-throughput screening to advanced imaging and telemetered cardiovascular assessments, we leverage the latest tools to enhance efficiency and quality, accelerating your path to clinical trials.
Advanced systems document every part of your study protocols, results, and regulatory requirements, making them accessible and supporting GLP compliance. Whether you are preparing for IND submissions or clinical trials, you can trust that our studies strictly adhere to regulatory guidelines and that the scientific data we produce meets the highest standards of quality, which is crucial for the success of your submissions to regulatory agencies.
Why Choose Altasciences for Your Preclinical Pharmacology Studies?
When you partner with Altasciences for your preclinical pharmacology needs, you gain access to a team of dedicated professionals committed to your success. Whether you are a biotech startup, a mid-sized biotech/pharmaceutical, or a large pharmaceutical company, our proven track record and commitment to quality results make us the ideal partner for your pharmacology needs.
In addition to our testing services, we provide expert consultation throughout the entire preclinical phase of your program to improve the overall design and outcome of your studies.
Contact us today to learn more about how we can help you achieve your drug development goals.
Preclinical Pharmacology Studies: FAQs
What is preclinical pharmacology and why is it important in drug development?
Preclinical pharmacology studies are the first step in a drug development program and occur before first in-human trials. The goal is to gather data so that we can predict how the drug will behave in humans. This includes its potential side effects, its overall safety, and efficacy. Key research areas of preclinical pharmacology include aspects of pharmacodynamics (PD), pharmacokinetics (PK), toxicology, safety pharmacology, efficacy studies, as well as elements related to drug formulation and delivery.
What types of preclinical pharmacology studies does Altasciences offer?
- In vivo pharmacology: Animal-based studies investigating the drug's effects on living organisms. These can include dose-response assessments involving acute functional effects, early toxicity evaluations, and physiological and behavioral studies to determine efficacy and safety.
- Pharmacokinetic (PK) studies: PK studies assess how a drug is absorbed, distributed, metabolized, and excreted (ADME) in animal models. This information is critical for determining safety margins for establishing dosing regimens in human clinical trials.
- Pharmacodynamic (PD) studies: PD studies examine the relationship between the drug dose and the pharmacological response, which may involve measuring biomarkers or physiological or behavioral effects in animals.
- Toxicology studies: Nonclinical safety studies of this type are conducted in animals to identify potential toxic effects. These studies are essential for assessing risk before initiation and during the progression of human clinical trials.
- Biomarker discovery and validation: Research to identify and validate biomarkers for use in nonclinical studies and clinical trials helping to understand the therapeutic mechanism of action and off-target effects.
How do pharmacokinetics (PK) and pharmacodynamics (PD) studies impact drug efficacy?
Pharmacokinetic (PK) studies play a crucial role in determining the efficacy of a drug by examining several key factors, including bioavailability. First, they help identify the optimal dose and corresponding tissue concentration needed to achieve effective drug levels at the site of action, considering relevant features of absorption and metabolism. These studies also help to determine how long it takes for the drug to reach its maximum concentration, steady-state levels, and elimination profile, which help guide the establishment of safe and effective dosing schedules.
Pharmacodynamic (PD) studies are pivotal in establishing the range of acute and chronic drug concentrations that are both effective and non-toxic. Specifically, these studies help establish dose-response relationships by defining how the drug’s dose correlates with the magnitude of its effect, enabling researchers to pinpoint the minimum effective dose and the optimal dose that maximizes benefits without causing adverse effects. PD studies also aid in identifying the therapeutic targets of a drug, such as receptors or enzymes, and understanding how the drug interacts with these targets to achieve the desired therapeutic outcomes.
What preclinical models are used to assess drug efficacy and safety?
Altasciences uses a variety of preclinical models to assess drug efficacy and safety, including in vitro cell-based assays, rodents (mice, rats), and non-rodent species (such as dogs, swine, and nonhuman primates). These models effectively simulate human biology, physiology, and states of disease.
What types of biomarkers are evaluated in preclinical pharmacology?
Biomarkers in preclinical pharmacology help assess drug efficacy, mechanism(s) of action, and potential toxicity. This can help provide valuable data regarding a drug’s biological activity before in-human trials. Some of these biomarkers include:
- pharmacodynamic markers (e.g., receptor binding, occupancy and functional activation, enzyme activity)
- genetic markers (e.g., gene expression changes)
- protein biomarkers (e.g., cytokines, growth factors)
- metabolic biomarkers (e.g., drug metabolites, endogenous responses to drug treatment)
What role does dose-response analysis play in preclinical pharmacology and toxicology studies?
In preclinical pharmacology and toxicology studies, the goal of dose-response analysis is to assess the relationship between the dose of a drug and its therapeutic or toxic effects. Additionally, studies designed to characterize dose-response profiles are used in preclinical research to help identify the minimum effective dose (MED), the optimal therapeutic dose range, and the maximum tolerated dose (MTD). These data help assist in the identification of safe and effective dose ranges to allow safe progression into human clinical trials and, ultimately, market licensure.
Related Resources
-
 PRECLINICAL RESEARCH SERVICES
PRECLINICAL RESEARCH SERVICES -
 ALTASCIENCES' FACILITIES
ALTASCIENCES' FACILITIES -
 PLANNING YOUR PRECLINICAL ASSESSMENT
PLANNING YOUR PRECLINICAL ASSESSMENT -
 PROACTIVE DRUG DEVELOPMENT SOLUTIONS—SMALL MOLECULES
PROACTIVE DRUG DEVELOPMENT SOLUTIONS—SMALL MOLECULES -
 PROACTIVE DRUG DEVELOPMENT SOLUTIONS—LARGE MOLECULES
PROACTIVE DRUG DEVELOPMENT SOLUTIONS—LARGE MOLECULES
Therapeutic Areas
Our deep expertise and capabilities in a broad range of therapeutic areas encompasses preclinical and early clinical studies for both small molecules and biologics. We can manage your entire program, as well as provide comprehensive support research services and bioanalytical expertise.