Ethnobridging Clinical Trials
SUPPORTING MULTI-REGIONAL DRUG SUBMISSIONS WITH ETHNOBRIDGING
Ethnobridging studies evaluate drug exposure differences between Asian and non-Asian populations by comparing the pharmacokinetics of the investigational drug across both groups. These early Phase I studies provide the data needed to include Asian populations in global safety and efficacy Phase II–III trials without repeating Phase I programs in those same regions.
We can help you accelerate this process with Asian ethnobridging studies conducted in North America, as early as your first-in-human trial—streamlining your development timeline and global regulatory strategy.
Why Choose Us for Ethnobridging? Because Expertise Matters
With over 20 years of experience and more than 250 ethnobridging studies completed, we the partner of choice for early-phase clinical research. Our expertise helps you accelerate development timelines and increase asset value through two flexible solutions:
- Standalone Asian Ethnobridging Study: Conducted in the U.S. after identifying target doses for your global program.
- Integrated Approach: Include Asian participants in first-in-human (FIH) studies or other pharmacokinetic (PK)-focused clinical pharmacology trials performed in the U.S.
We conducted the largest ethnobridging study to date, which resulted in a U.S. label update recommending a 50% dose reduction for Asian patients. This insight gained early in development, allowed our sponsor to optimize dosing, and mitigate risk ahead of later-phase trials and regulatory submissions across Asian regions.
Consult our Fact Sheet for more information on our ethnobridging clinical trial service capabilities.
ACCELERATED GLOBAL DRUG DEVELOPMENT TIMELINE
Our approach to ethnobridging clinical trials begins in Phase I and focuses on two key advantages:
- Including Japanese, Chinese, Taiwanese, and Korean participants in North American trials following ICH guidelines, which produces data accepted across most Asian countries.
- Studying all required populations simultaneously, instead of waiting for non-Asian trials to finish before beginning bridging studies in Asia.
By conducting ethnobridging studies this way, you can significantly shorten drug development timelines in Asian regions compared to traditional approaches. This strategy helps sponsors safely speed up regional development and regulatory submissions worldwide.
For sponsors intending to develop a drug in Asian markets simultaneously, the development plan would follow a structure similar to that shown in the figure below.
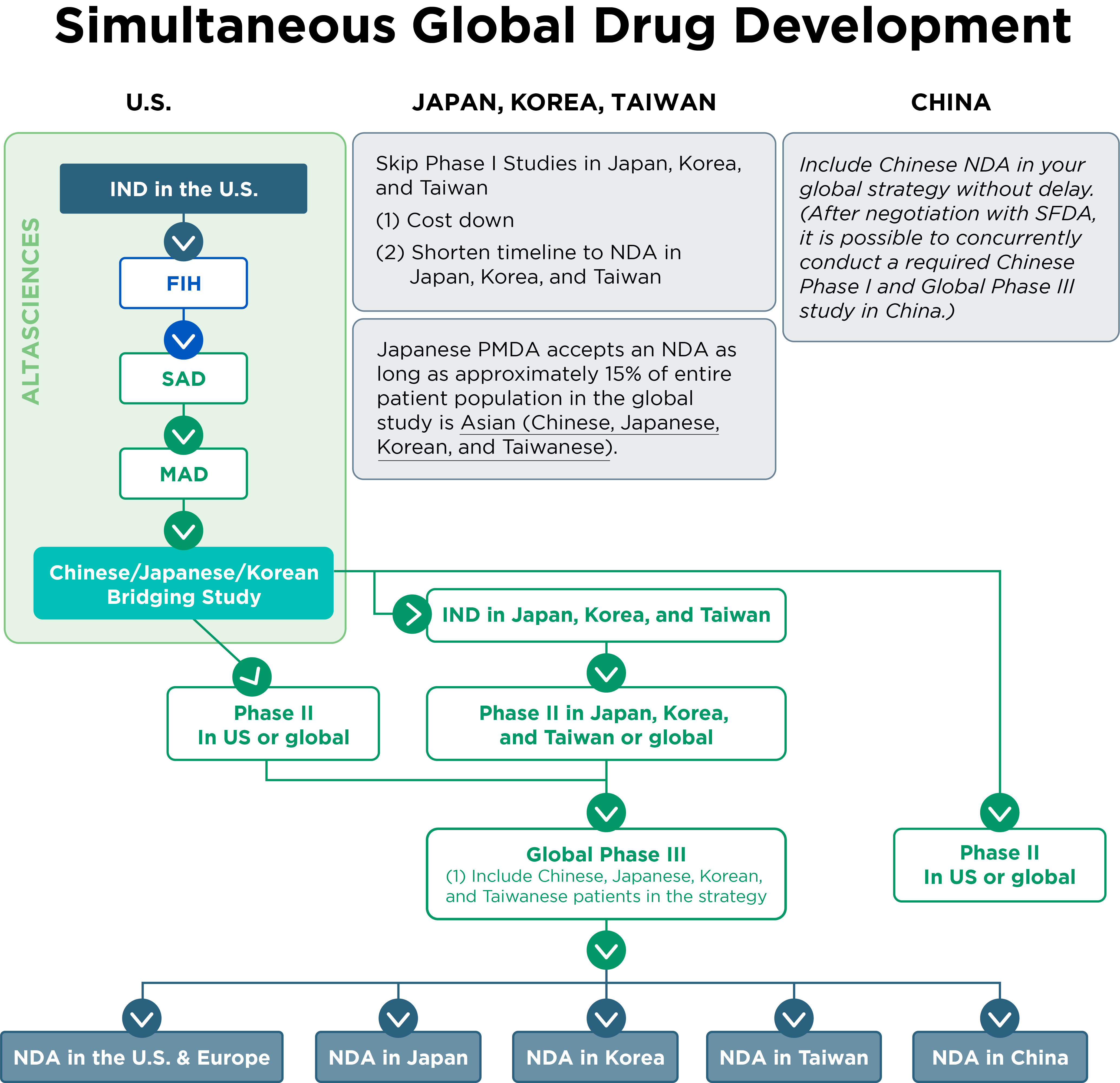
- The PMDA in Japan will accept an NDA submission if approximately 15% of the total patient population in a global study consists of Asian participants (Chinese, Japanese, Korean, and Taiwanese).
- Based on discussions with the Chinese State Food and Drug Administration (SFDA), conducting the required Chinese Phase I study concurrently with a global Phase III study in China is possible.
RECRUITMENT OF ETHNIC POPULATION
Altasciences Los Angeles Phase I CPU is strategically situated within a large metropolitan area and is able to recruit from a diverse ethnic population. We have a dedicated, multilingual Asian recruitment and outreach department to liaise with our participants.
- We recruit an average of ~800 Asian participants yearly
- We have over 12,000 Asian participants in our database
- We have multilingual recruiting, marketing, and clinical operations staff
TURNKEY SOLUTION FOR ETHNOBRIDGING CLINICAL TRIALS
We offer a turnkey solution for conducting ethnobridging studies, managing every aspect of your trial from start to finish. Our comprehensive services include:

Study Design:
We collaborate with you to develop an ethnobridging study that meets scientific objectives and aligns with global regulatory expectations.

Regulatory Consulting:
Our team is well-versed in international and regional regulatory requirements, including those of the PMDA (Japan) and SFDA (China). We guide you through the ethnobridging regulatory pathway, ensuring that elements outlined in ICH E5 and ICH E17 are appropriately incorporated into the study design.

Clinical Conduct:
Our state-of-the-art clinics are fully equipped to conduct your ethnobridging study, with expert teams managing dosing, sampling, and intensive monitoring.
We offer specialized services for ethnobridging, including:
- Native-speaking recruitment teams for effective outreach and engagement.
- Language and cultural support to ensure participant comfort throughout the trial.
- Ethnic-specific meals when permitted by protocol.
- Multilingual study documents, including informed consent forms (ICFs), to ensure clarity and understanding.

Comprehensive Monitoring:
Real-time tracking of pharmacokinetics (PK) and pharmacodynamics (PD) data, safety assessments, and adverse event reporting ensures compliance with regulatory standards and maintains data integrity.

Bioanalytical Services:
Our co-located bioanalytical laboratories provide precise and timely PK analysis. Leveraging either custom or established methods, we deliver reliable data that support faster decisions and robust regulatory submissions.

Data Services and Reporting:
From data management and biostatistics to final reporting, we ensure accurate, high-quality results delivered on schedule.
We’re fully equipped to conduct your ethnobridging strategically, meet enrollment targets, and deliver dependable results on time.
ETHNOBRIDGING CASE STUDY
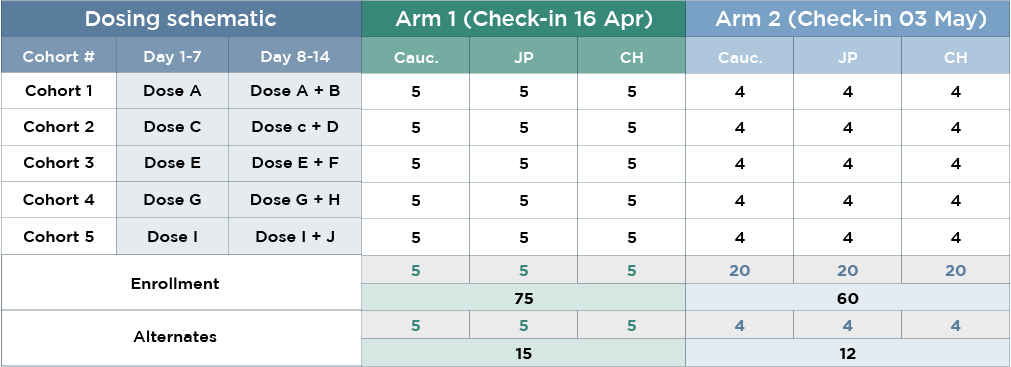
This ethnobridging study evaluated the multiple-dose pharmacokinetics and safety of a co-administered drug regimen across Han Chinese, Japanese, and Caucasian populations, with rapid enrollment and efficient execution.
- 45 Han Chinese, Japanese, and Caucasian subjects (135 total)
- Quick recruitment hastened timelines for full completion
- Screening duration: 35 days
- Enrollment duration: 17 days
Related Ethnobdirging Resources
Ethnobridging in Phase I Clinical Trials
Check out our webinar on how ethnic differences can impact the bioavailability of your drug candidate and how to design your cross-regional clinical development program.
The Altascientist: Shortening Drug Development Timelines With Asian Ethnobridging Trials
Read this issue of The Altascientist to learn about strategies and case studies for including ethnobridging in your early clinical development plans and safely accelerating regulatory acceptance of data worldwide.
Consult our fact sheet to learn about our ethnobridging capabilities and view case studies.
Protocol Design Concepts in Phase I Ethnobridging Trials
Explore an overview of Phase I ethnobridging trial design concepts, highlighting PK/PD modeling, safety assessments, and global treatment alignment to streamline drug development.
Supporting Global Clinical Development
Discover how ethnobridging, as recommended by the ICH E5 guidelines, addresses historical challenges in global drug approval, particularly in Asian countries.
Ethnobridging Clinical Research – FAQs
What is the purpose of Asian ethnobridging?
Developing drugs for the Asian market may require that Phase I clinical trials be repeated in regions outside North America or Europe, to determine whether the pharmacokinetics of the investigational drug are equivalent in different ethnic groups. Asian ethnobridging is a strategy to demonstrate biosimilarity of drug products between Asian and non-Asian populations by comparing pharmacokinetics of the investigational drug in both ethnic groups, considering intrinsic and extrinsic factors.
How does ethnobridging affect global drug development?
Conducting ethnobridging clinical trials during Phase I can reduce drug development timelines by the number of years typically needed to complete clinical development in the target region, as compared with North America or Europe, enabling faster global access to needed medications.
What are the regulatory requirements for ethnobridging trials?
- The PMDA accepts ethnobridging trials conducted in the United States.
- A PMDA consultation is an excellent way to ensure compliance and establish a binding agreement. The consultation is not free and should be scheduled at least two months in advance, but it helps ensure that all stipulations of ICH E5 are met.
- Studies can vary greatly depending on several key factors, including the type of medication, regional ethnicity, medical practice, drug class, and clinical experience. Once these factors have been evaluated, a bridging strategy can be developed, and clinical research can begin.
What are the qualifications of the participants of ethnic specific groups?
For studies requiring the participation of specific ethnic groups, the only necessary qualification is that they can prove ancestry on both sides of their family. Our past experiences have taught us that genetic compliance is the only predetermined factor. Extrinsic factors, such as how long the individual has been living in the United States, do not change the genetic factors examined in ethnobridging trials.
What is a typical cohort size for Ethnic Bridging?
The typical cohort size varies based on the needs of the specific study. Altasciences has conducted studies with 135 subjects (45 Han Chinese, 45 Japanese, and 45 Caucasian). We’ve also conducted many studies where fewer (8-12) were required. In general, cohort size ranges from trial to trial and requires the CRO to understand the developer’s specific development and commercialization needs.
What is Altasciences’ suggested model for accelerating drug development in Asia?
To eliminate the need for separate Phase I trials in the target region, we propose conducting Phase I development, including an ethnobridging portion for the intended Asian region, in a single location, such as the U.S. Based on these results, we would proceed directly to Phase II-III development in the target region. To accelerate your trials even more, we would integrate the ethnic group into the First-In-Human study rather than having a separate study for ethnobridging, so that you can determine safety, tolerance, and PK/ PD across doses and ethnic groups.
When Asian countries approve a drug, is there a delay in approval from Western Countries?
In general, delays have traditionally taken place during approval in Asian countries, whereas countries in the EU and North America tend to reach faster conclusions. As of late, Taiwanese, Japanese, and Korean health authorities have been working together to streamline processes, sharing data and procedural information to ensure fewer delays. Globally, with the increase in ethnobridging trials and formal cooperation and communication between Asian countries, development delays leading to health crises are becoming far less common.




