A Snapshot of the Webinar “Development of a Cell-Based Assay”
Cell-based assays are usually used to represent the mechanism of action (MoA) of the administered drug. Throughout various stages of drug development, they may be used for different purposes. These include detecting the presence of neutralizing antibodies (NAbs) and evaluating a drug’s potency. In Altasciences’ webinar, “Development of a Cell-Based Assay,” Dr. Danielle Salha, Senior Director, Immunochemistry & Immunology, Ligand Binding Assays, discusses key points to consider when developing a cell-based assay.
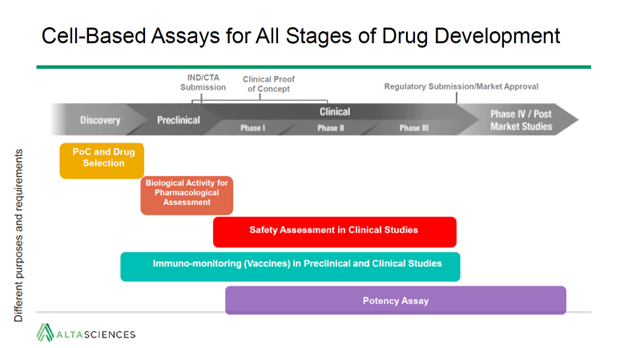
Here are three of the main takeaways:
1. Optimize your NAb monitoring method
Using cell-based assays to monitor NAbs serves a dual purpose. In addition to impacting safety by causing an autoimmune reaction, NAbs can compromise efficacy by neutralizing drug activity.
In order to obtain meaningful results, use Design of Experiment (DoE) during method development to plan and conduct your cell-based assay. A DoE helps to ensure a dynamic range large enough for an adequate level of sensitivity in your study results. Critical parameters to be evaluated in designing your experiment include cell selection, cell density, seeding time, minimum required dilution, and drug concentrations. Optimizing these and other parameters by testing them simultaneously will enable you to arrive at the best possible combination for your assay.
2. Select the appropriate assay
NAbs can be tested using a cell-based assay (CBA) or a non-cell-based assay like competitive ligand binding (CLB). The FDA guidance recommends a CBA format unless there is scientific evidence in favor of a different method.
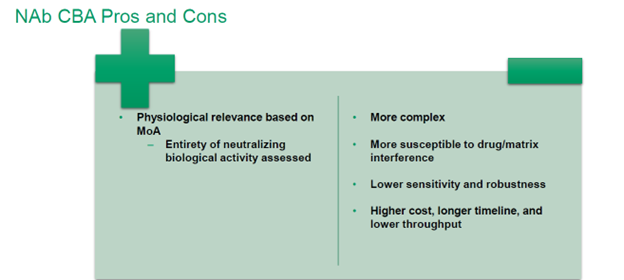
Choose a CBA NAb assay for therapeutic proteins with a complex MoA representing a sequence of linked events that lead to biological activity, and/or following a high level of safety risk. While a competitive ligand binding assay only measures a single receptor, a cell-based assay can mimic the complete series of steps leading to cell activation. Where the protein’s MoA is unknown, it is usually better to err on the side of caution and use a CBA.
3. Use a common cell-based assay for both NAb and potency method development
NAb and potency assays serve different purposes. NAb assays yield qualitative results that determine whether a participant’s immune system is impacting drug safety and efficacy. On the other hand, potency assays are quantitative measures of a drug’s biological activity.
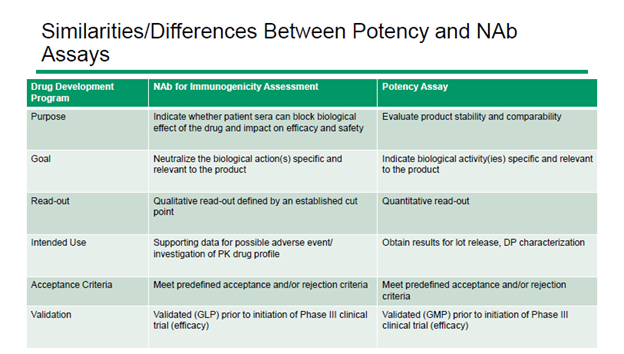
In spite of their difference, there are areas of overlap between NAb and potency requirements. As a result, the same cell-based assay can sometimes be used for both applications. For very complex assays, this can lead to a time saving of up to four months.
Starting with parameters common to each type of assay, the dynamic range can be specified for both.
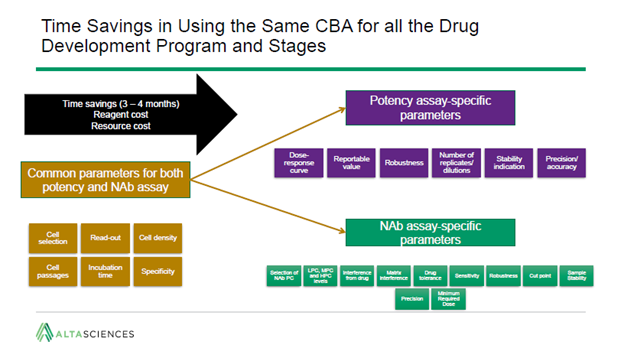
Using the same cell-based assay for both applications can also allow for correlation between different data sets. For instance, the same biological activity may be responsible for an effect on both immunity and potency. In addition, the same risks that make a Phase I safety assessment a regulatory requirement will likely also necessitate later potency testing.
With expertise in both CBAs and non-cell-based assays, Altasciences’ team can choose the appropriate assay or combination of assays for your studies. You will get the meaningful data you need to meet your regulatory requirements while maximizing time savings and minimizing costs. To learn more, watch the on-demand webinar or contact us to get in touch with one of our experts.



