Highly Potent API (HPAPI) Handling
Oncology research, cancer treatments, and other targeted therapies, have made highly potent active pharmaceutical ingredients (HPAPIs) a rapidly growing segment in the pharmaceutical industry worldwide. There are currently thousands of HPAPIs in development that promise lower dose requirements and/or fewer side effects. Handling of these compounds requires expertise and specialized equipment to take them from formulation to manufacturing for clinical supply and eventually, to market.
Our Expertise
We have decades of experience formulating, developing, and manufacturing drug products containing HPAPIs, within our facility’s high-potency/Grade C and D manufacturing and handling areas. Our highly trained experts have handled numerous projects, including analytical testing and method development, qualification, and validation. We provide cost-effective process scale-up for your projects, from prototype formulations to commercial scale batches, using a range of formulation techniques and dosage forms, including microparticle formulations and nanomilling.
QUICK CHAT: This short video provides a quick overview of our solutions for highly potent compounds (HPAPIs), including the seamless shipping and handling of controlled substances.
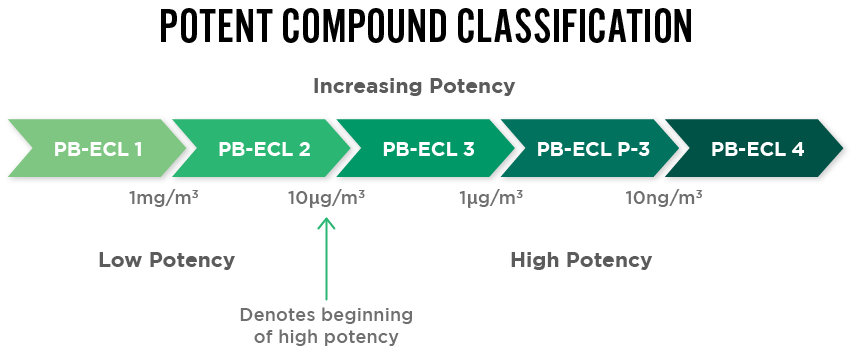
API Classification
New APIs undergo robust evaluation to determine their classification level for safe handling procedures and clearance limits. Performance-based exposure control limit (PB-ECL) is then used to correctly classify the compound (Class 1 to Class 4).
cGMP Facility
Our 64,000-square-foot cGMP facility is designed and equipped to meet the challenges associated with the complex handling of HPAPIs and other controlled substances with high toxicity levels, including:
- Dedicated high-potency/Grade C and D manufacturing and handling areas
- Registrations and licenses: FDA drug establishment registration, manufacturer DEA license (Schedules I-V and List 1), FDA food facility registration
- Advanced equipment used for HPAPI handling and manufacturing , including:
- Kettles
- Mills
- Peristaltic pumps
- Heater/chillers
- Vibroscreens
- Vial crimpers
We have strict safety and security processes in place for the containment of potent compounds to minimize risk of exposure, and ensure our facility and personnel are adequately protected at all times:
- Stringent safety and cleaning protocols for HPAPI containment, with 100% analytical verification for room clearances
- Total enclosure processes to reduce employee exposure
- Rooms designed with one-way traffic flow to minimize and/or eliminate compound transfer
- Personal protective equipment (PPE), as well as respirators or powered air-purified respirators (PAPRs)
- Engineering controls for potent compound control, including:
- HEPA filters for superior air filtration
- Magnehelic® gauges
- Rees building monitoring system
 PODCAST: Altasciences’ experts present our potent handling capabilities, including a general overview of our facility and services, processes, equipment, and compound classifications.
PODCAST: Altasciences’ experts present our potent handling capabilities, including a general overview of our facility and services, processes, equipment, and compound classifications.
Have a highly potent API that needs to be developed? We can help. Send us a message.